____ SOILS, A COMPLEX LIVING ECOSYSTEM ___
Part 1
UN ECOLOGICAL APPROACH TO SOIL ANALYSIS
Soil ecology is the study of the interactions among soil organisms, and between biotic and abiotic aspects of the soil environment. It is particularly concerned with the cycling of nutrients, formation and stabilization of the pore structure, the spread and vitality of pathogens, and the biodiversity of this rich biological community. Ecological concepts are informative for edaphological studies on how soil properties influence the natural or cultivated plant communities in an area. The population dynamics and structure in plant communities depends upon the physico-chemical environment and its associated soil foodweb, which concentrates in the rhizosphere around growing plant roots and in the detritusphere containing the fresh and decaying plant litter. Biological activity of the plant-soil foodweb is controlled by positive and negative feedbacks that arise from mutualistic, benevolent, competitive, predatory and antagonistic interactions. The soil matrix is arranged with pores containing an air-water mixture and solids organized in porous organo-mineral aggregates. The persistence of soil structure depends upon geomorphic processes and pedogenesis, and is inherently dynamic and spatially heterogeneous in response to environmental disturbances. As soil is part of ecology, our understanding of complex soil processes is enriched by viewing it from the perspective of ecologists.
Interest of the ecological approach
Ecologists study the relations of organisms to one another, and to their physical surroundings. Ecologists consider soil to be an ecosystem with living and non-living components that interact with each other. Soil life includes permanent inhabitants like microorganisms, nematodes and earthworms, as well as plants, insects and burrowing mammals that spend all or part of their life in the soil. The genetic diversity, physiological functions, population dynamics and community structure of these organisms is of interest to ecologists. These subjects can be studied for a single organism or in the soil foodweb, a framework describing how energy and materials are transferred from one trophic level to the next within the soil environment. Non-living components of the soil ecosystem include the rocks and minerals, organic matter, water and air that create the physical soil structure. The arrangement of non-living components determines the habitat of soil organisms and their biological activity. Together, the biotic and abiotic components of the soil ecosystem contribute to ecological functions such as net primary production, biogeochemical reactions, gas exchange and hydrologic cycling. Thus, soil is part of ecology.
An ecological perspective enriches our appreciation of soil science. Ecologists are interested by the spatial and temporal dynamics of ecosystems, and the underlying causes of this variation. Soils of the world are inherently variable because of they formed from complex geological processes across landscapes with unique climatic and organismal influences, over unequal periods of time. Even if we constrain our study to 1 m2 of a soil profile extending from the soil surface to the bedrock, we will see remarkable spatio-temporal heterogeneity in the biological activity and structure of the soil matrix. This is because soil organisms are more abundant and more active near the surface, around roots, close to fresh detritus, and in well-aerated pores. We explain why these areas are preferred niches for soil organisms, and how their presence in these influential spheres maintains the soil structure. Temporal fluctuations in soil are caused by disturbances, which vary in intensity, frequency and scale. A light rainfall is an innocuous disturbance that changes the air-water balance in soil pores and stimulates the soil chemical, biochemical and biological processes for several hours. Wildfires, floods and prolonged droughts are intense disturbances that can disrupt and change the trajectory of ecological succession in the soil ecosystem for years to decades. We will discuss the concept of succession in the context of disturbance, explaining how such events alter the interactions among organisms and the soil abiotic components.
Soil spheres, their biological activity and ecological functions
Looking across a typical agricultural field, grassland or forest area, an ecologist will inevitably see variation in soil conditions, plant communities, hydrologic processes and gas exchanges. This arises due to small-scale variation in the biotic and abiotic features of the ecosystem that create niches for soil organisms. Locations occupied by more soil organisms are known as “hot spots” and they may support more biological activity at “hot moments” in time. Here, we describe the characteristics of three soil spheres – the rhizosphere, the detritusphere and the porosphere (Fig. 1).
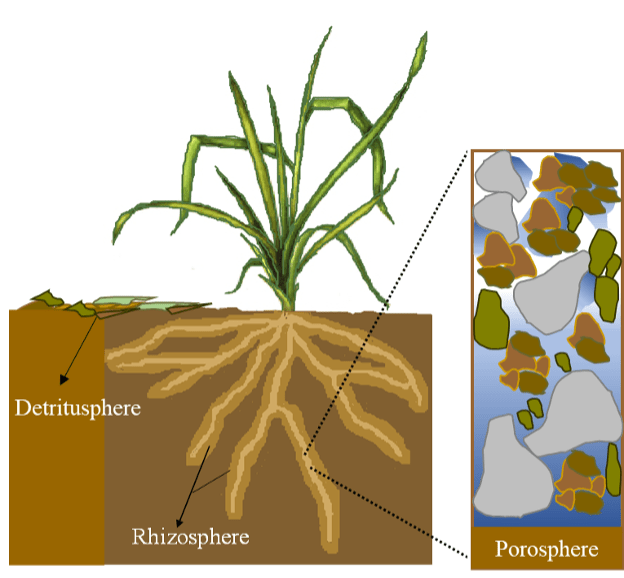
Fig. 1. Soil biological activity is concentrated in soil spheres – in the rhizosphere around plant roots, in the detritusphere containing fresh, partially and well-decomposed organic residues, and in the porosphere, the air- and water-filled space around soil solids (fragments of organic matter, sand, silt and clay, as distinct particles and agglomerated in soil aggregates).
Rhizosphere and its ecological significance
The rhizosphere is defined as the soil that is in contact with plant roots. The rhizosphere is created when the primary radical emerges from a germinating seed. The rhizosphere expands every day, following a diurnal pattern. Roots of thale cress seedlings (Arabidopsis thaliana) elongate at a rate of 55–120 μm h-1, with the maximum extension rate about 1–2 h after dawn (Yazdanbakhsh and Fisahn 2010). During the day, root extension slows and stabilizes, with minimal growth rates during the last 3 h of daylight and just after dark. Root elongation resumes during the night, peaking after dawn on the next day (Fig. 2).
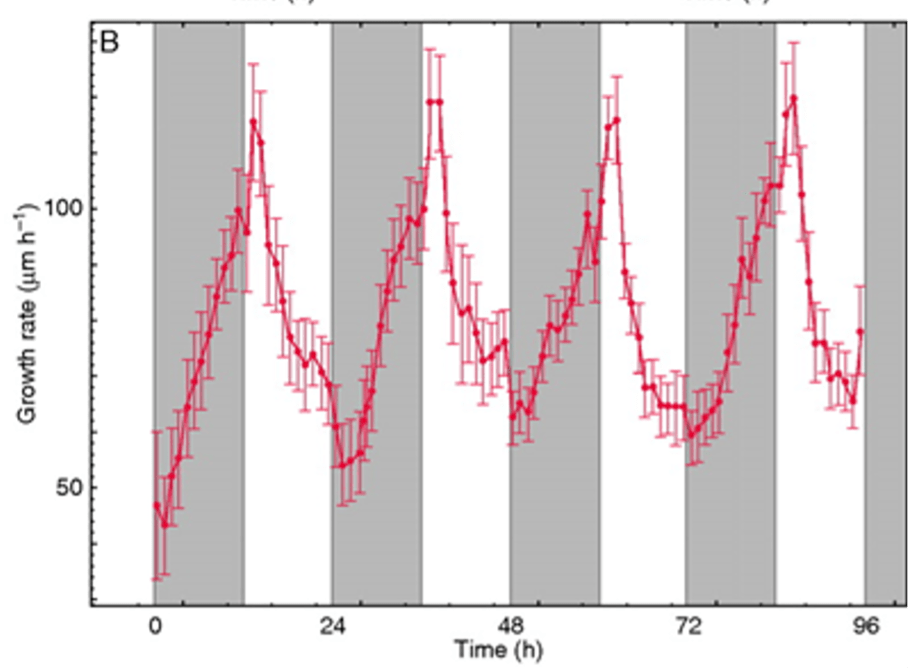
Fig. 2. Root extension profiles of Arabidopsis thaliana L. wild-type (Col0) growing at 21 ºC in 12 h light /12 h dark cycles. Eleven-day-old seedlings were monitored for 4 d, and the average root extension rate (n=6, with standard error bars) was calculated from displacement of the root tip in consecutive time periods. The temporal pattern of daily root growth shows the minimum growth rate after dark, increasing during the night and reaching a maximum within 1–2 h after dawn, and gradually declining during the day. From Yazdanbakhsh N, Fisahn J (2010) Analysis of Arabidopsis thaliana root growth kinetics with high temporal and spatial resolution. Ann Bot 105(5):783–791. doi: 10.1093/aob/mcq048
The spatial extent of the rhizosphere depends upon the plant species and soil conditions. In the Kalahari desert, the root system of Shepherd’s tree (Boscia albitrunca) extends >70 m, apparently to access subterranean water in a region that receives <200 mm of annual rainfall (Fan et al 2017). However, 95% of plant roots are in the 0–40 cm layer of the soil profile in the Mojave desert (Wallace et al 1980). Desert shrubs, including Atriplex sp., Lycium sp. and Ambrosia dumosa, grow multiple taproots in the top 10 cm and produce more small lateral roots in the 10–30 cm. The shallow rooting depth is related to the scarcity of rainfall (~100 mm per year) and the presence of an impermeable caliche layer, 30–50 cm below the soil surface. Besides the physical barrier to rooting caused by soil compaction and rocky layers, roots also avoid growing in waterlogged areas of the soil profile. Plant roots require oxygen for cellular respiration, and a soil-air oxygen pressure <10 kPa inhibits root extension (Sønderholm and Bjerrum 2021). Roots also detect chemical gradients in the environment, which leads them to proliferate in soil layers with favorable pH, low salt content and sufficient nutrient concentrations. Some plants produce cluster roots, a number of tightly grouped determinate rootlets that initiate and grow in a coordinated manner in response to low concentrations of phosphorus, nitrogen and iron in the rhizosphere (Shane and Lambers 2005). Once a cluster has developed, it releases carboxylates at a very fast rate, solubilizing phosphate and other ions to increase the amounts absorbed by the roots (Fig. 3).

Fig. 3. Compound cluster roots of Banksia grandis grown in a hydroponic system with extremely low phosphate supply (≤1 μM). Young developing cluster roots (black arrow) are less dense than the older, mature cluster roots (white arrow). The white bar is 20 mm long. From Shane MW, Lambers H (2005) Cluster roots: A curiosity in context. Plant Soil 274:101–125. doi: 10.1007/s11104-004-2725-7
The rhizosphere is a unique environment that attracts and supports the growth of more soil organisms than the surrounding soil. Some organisms reach the rhizosphere passively, as water flowing towards the root due to evapotranspiration carries dormant bacterial cells, fungal spores, and encysted protists and nematodes to the root surface. Such passive dispersal is common, since up to 80% of the microbial cells in soil are dormant at any point in time (Blagodatskaya and Kuzyakov 2013), and soil bacterial communities that disperse into substrate-rich environments are more resilient than those that do not (Sorensen and Shade 2020). Roots release a multitude of organic substrates into the rhizosphere. Substrates include cellular debris and mucilage that are physically abraded during root extension, and molecules like organic acids, phenolics, peptides, vitamins and hormones that are synthesized and secreted to attract and nourish soil organisms. This rhizodeposition represents a net carbon flux from the plant root into the soil that is 17–40% of the total carbon from photosynthesis during the plant lifespan (de la Fuente Cantó et al 2020), with a greater proportion of photosynthates allocated to the roots of younger than older plants and when plants are stressed due to unfavorable weather, herbivory and infectious agents.
Signal molecules are a class of root exudates that act as infochemicals for soil microorganisms and soil fauna to detect the presence of roots. Signals exchanged between roots and soil fauna create the rhizobiome, a community of organisms that live on the external and internal root surfaces. Plants can deliberately synthesize and secrete specific compounds that select for and assemble the rhizobiome (Bever et al. 2010). This interaction is well-described for plants associated with bacteria that catalyze biological nitrogen (N2) fixation, a reaction that uses N2 from the atmosphere together with energy, water, and H+ ions to produce ammonia (NH3) and hydrogen gas (H2):
N2 + 16 ATP + 16 H2O + 8e- + 8H+ —-> 2NH3 +H2 + 16ADP + 16Pi [1]
Bacteria capable of this reaction possess the nitrogenase enzyme, a catalytic molybdenum-iron protein (MoFeP) and reductive iron protein (FeP) that complete electron and proton transfer processes through conformational changes in the ATP-dependent FeP–MoFeP complex.
In legumes of the Fabaceae family, biological N2 fixation occurs in root nodules occupied by the bacterial symbiont, rhizobia belonging to the genera Rhizobium, Bradyrhizobium or Ensifer (also called Sinorhizobium). The genetic basis for the legume-rhizobia symbiosis is summarized for the model legume Medicago truncatula (Gautrat et al 2021) Nitrogen-deficient roots of M. truncatula produce a peptide hormone from the C-terminally encoded peptide (CEP) family that is perceived in the shoots by the receptor Compact Root Architecture2 (CRA2). This combined root-shoot pathway controls the length of the primary root and determines the extent of lateral root development, also influencing the angle that lateral roots grow from the primary root. Rhizobia in the rhizosphere trigger a cytokinin signaling pathway recognized by the cytokinin receptor CRE1 and the Nodule Inception transcription factor that initiate nodulation. The rhizobia pathway interacts with signals exchanged by CEP-CRA2, leading to nodulation when the soil nitrate (NO3−) concentration is sufficiently low (Gautrat et al 2021). These genetic controls make the legume-rhizobia symbiosis a highly efficient way to fulfill the nitrogen requirements of grain legumes like lupin, chickpea, lentil and bean, which obtain 41 to 71% of the nitrogen required to produce aboveground biomass from biological N2 fixation (Palmero et al 2022).
Benevolent interaction between plants and bacteria
Non-legume plants also benefit from biological N2 fixation by root endophytes (bacteria that become resident in root tissues and live there for at least part of their life cycle, without causing disease) and free-living root-associated bacteria. Known collectively as diazotrophs, these non-symbiotic N2 fixing bacteria do not form nodules but do possess a functional nifH gene that encodes for the nitrogenase enzyme. Root endophytic diazotrophs was first detected in sugarcane in the 1970s (Gluconacetobacter spp., previously Acetobacter diazotrophicus), with additional discoveries in the 1980s of Azospirillium living inside the roots of wheat and sorghum (Sorghum spp.). Since then, hundreds of bacteria and archaea were found to have N2 fixation capacity in cultivated true grasses (Poaceae family, formerly known as Gramineae). Some sugarcane varieties derive up to 70% of their nitrogen requirement from biological N2 fixation through their association with Gluconacetobacter diazotrophicus. Rice, wheat, barley and other cereals also obtain nitrogen for metabolic processes from diazotrophs that colonize their roots, stems and leaves. Initial surveys of diazotrophs were based on selective, N-limited growth media. Recent advances in metagenomics improve our ability to detect putative N2 fixing organisms possessing the nifH gene. For example, rice supports diazotrophs with nifH sequences from the alphaproteobacteria (Thiobacillus-like, Methylocystis), betaproteobacteria (Azoarcus, Azovibrio, Herbaspirillum), deltaproteobacteria (Desulfovibrio), gammaproteobacteria (Klebsiella-like, Azotobacter, Geobacter sulfurreducens) and the Firmicutes (related to Clostridium and Heliobacterium). In sorghum, the proteobacterial diazotrophs had nifH sequences related to bacteria from the orders Bacillales, Burkholderiales, Diazotrophs, Enterobacteriale, Rhizobiales, Rhodospirillales and Sphingomonadales (Rosenblueth et al 2018). Although cereal crops, through their interactions with root endophytes, have potential to acquire N2 from the atmosphere, it falls short of suppling enough nitrogen to meet grain yield targets. Ongoing research into diazotroph-plant partnerships is helping farmers to understand how N2-fixing root endophytes may be used to meet the nutritional needs of cereal crops.
Besides the benevolent bipartite interaction between plants and N2 fixing bacteria, the rhizosphere is host to complex multi-trophic interactions (Fig. 4). Bacteria and fungi attracted to the root surface, or colonizing the root tissues as endophytes, including mycorrhizal fungi, are part of the soil foodweb. Bacteria (primary consumers) typically attach and proliferate as biofilms using substrates released through the root epidermis. Arbuscular mycorrhizal fungi are root endophytes and obligate root symbionts for the majority of terrestrial plants, also considered to be primary consumers due to their reliance on photosynthates (from 15–30% of net primary production is transferred from roots to mycorrhizal fungi; Chapin and Eviner 2014). Root feeding nematodes are also considered to be primary consumers because they get their nutrition directly from the root tissues. Secondary consumers could be fungivorous bacteria, such as those growing in biofilms on the surface of arbuscular mycorrhizal fungi, as illustrated for Rhizoglomus irregulare colonized by Burkholderia anthina (Fig. 4; Taktek et al 2017). Secondary consumers may use antibiotics, other growth-inhibitory or toxic compounds to halt fungal growth, or they may infect fungi with viruses that cause cellular lysis. Tertiary consumers are predatory protists and nematodes (e.g., bacterivorous and fungivorous nematodes) that obtain nourishment by engulfing, crushing or sucking the cytoplasm from damaged bacterial and fungal cells. These interactions may be positive or negative for the soil foodweb. For the primary consumers, root exudation supports rapid microbial growth (positive feedback) but the root surface is a finite space and competition among diverse populations for resources will cause a negative feedback that regulates the size of the rhizobiome. On the other hand, predation by tertiary consumers appears to be a negative feedback, since it removes microbial biomass. However, predation can act as a positive feedback because removal of older microbial biomass allows for rejuvenation of microbial population with younger, biologically active cells. Predation may also remove potentially infectious plant pathogens before they cause plant disease. Thus, the rhizosphere is a hot spot where plants interact with benevolent and antagonistic soil organisms whose activities are orchestrated, in part, by trophic relationships in the soil foodweb.
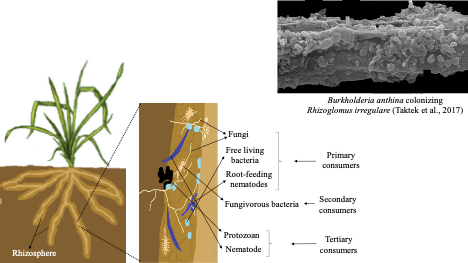
Fig. 4. Soil foodweb, showing the primary consumers (bacteria, fungi, root-feeding nematodes) and higher trophic groups, including the secondary consumers (fungivorous bacteria) and tertiary consumers (protozoan, nematode). The photograph is a scanning electron micrograph of Burkholderia anthina bacterial matrix formed on the hyphae of mycorrhizal fungus Rhizoglomus irregulare, illustrating the exopolysaccharides as horizontal layers and cocci-shaped bacterial cells of the biofilm, coating the fungal hyphae. From Taktek S, St-Arnaud M, Piché Y, Fortin A, Antoun H (2017) Igneous phosphate rock solubilization by biofilm-forming mycorrhizobacteria and hyphobacteria associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza 27:13–22. doi: 10.1007/s00572-016-0726-z
Detritusphere and its ecological significance
The detritusphere is the layer of dead and decaying litter originating from plants. In agricultural, grassland and forest ecosystems, the detritusphere is formed by the residues that remain after the crop is harvested or by the natural deposition of senesced leaves, stems and woody debris on the soil surface. When undisturbed by machinery and human activities, the detritusphere forms a distinct O horizon containing undecomposed, partially, or highly decomposed litter (leaves, needles, twigs, moss and lichens) above the mineral soil horizons. The detitusphere is augmented by grazing animals, which eat plants and defecate the non-digestable fibers on the soil surface. Humans add to the detritusphere by spreading mulch, compost, sewage sludge and municipal residuals, all of which contain plant residues that are fresh or decomposed at the time of application. Whether these residues are left on the soil surface or tilled into the soil profile, plant litter is an attractive substrate for soil organisms because it is moist, high in organic matter and rich in nutrients.
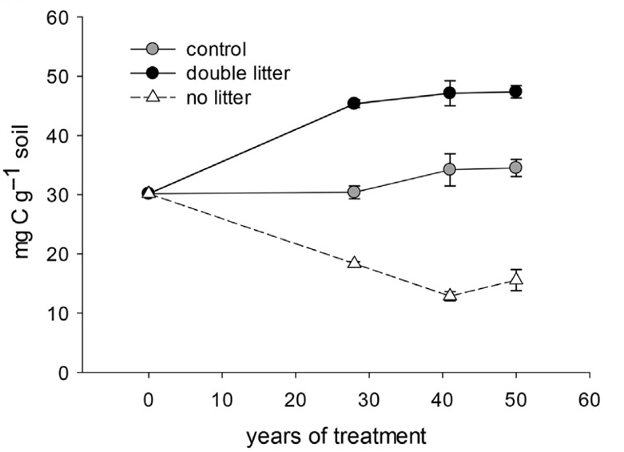
Fig. 5. Soil organic carbon concentration (mg C g-1 soil) in the 0–10 cm (A horizon) of Noe Woods, University of Wisconsin Arboretum, United States. Experimental treatments were established in 1956 and measured in 1984, 1997 and 2006. The control received the natural above- and below-ground litter inputs but coarse woody debris (>1 cm dia.) was removed, and seedlings and herbaceous material were removed. Double litter received the above ground leaf or needle inputs that was collected from the no litter plots. The no litter treatment involved removing aboveground inputs during autumn senescence and periodically throughout the year. Values are the mean ± 1 standard error (n = 4). From Lajtha K, Bowden RD, Crow S, Fekete I, Kotroczó I, Plante A, Simpson MJ, Nadelhoffer KJ (2018) The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci Total Environ 640–641:1112–1120. doi: 10.1016/j.scitotenv.2018.05.388.
Litter inputs contribute to soil biogeochemical cycling, since soil organisms use the complex organic substrates contained in plant residues for energy and nutrition. The amount of litter returned to the soil each year is greater in biomes with higher net primary production (e.g., tropical forests and grasslands) than in ecoregions where less plant biomass is produced. In general, the detritus comes from litterfall (about 10–30% of the net primary production), but also includes root inputs from rhizodeposition and fine root turnover, an additional 10–40% of net primary production (Chapin and Eviner 2014). Plant litter that enters the detritusphere is part of soil biogeochemical cycling, but it is also critical to maintain the soil organic matter and replenish soil fertility in the long-term. The detrital input and removal treatment (DIRT) project at forest and grassland sites in the United States illustrates the importance of plant litter inputs to maintain the soil carbon level. Doubling the aboveground litter inputs (Double Litter) increased by 1.5 times the soil organic carbon concentration in the 0–10 cm layer after 30 yr, whereas excluding the aboveground litter (No Litter) reduced the soil organic carbon by 50% after 30 yr (Fig. 5). Similar reductions in the soil organic carbon occur when roots are excluded or when no aboveground litter and roots are returned to the soil (Lajtha et al 2018). Returning plant litter to the soil will maintain the soil organic carbon, but it does not necessarily lead to a linear gain in carbon sequestration because soil mineralogy and microbial processes influence the amount of carbon retained in the soil-plant system.

Fig. 6. Earthworm midden created by Lumbricus terrestris L. Earthworms collect plant litter and partially bury it in the mineral soil layer, leaving a relatively bare soil surface around the midden in annually cropping agroecosystems. Photo by the authors.
Organic matter dynamics in the detritusphere is also determined by the physical and chemical properties of plant litter. Fresh inputs are coarse, intact pieces of leaves, stems, branches and lateral roots that must be physically reduced in size for further decomposition to occur. Soil invertebrates like termites, ants and earthworms will chew, tear and dislodge fragments of the plant debris. These fragments are often collected and stored in the nests or middens of these animals, where they are partially buried by soil minerals and colonized by soil microorganisms (Fig. 6). Once partially decomposed, the organic residues are consumed and redistributed by soil macrofauna and mesofauna, including the soil mites, collembola and other insects. These organisms are considered to be engineers in the soil foodweb, because they make organic substrates available for the use of primary consumers. Mechanical breakdown of fresh plant litter releases soluble ions and sugars that were contained in conductive tissues and vacuoles of cells. These readily metabolizable substrates support the growth of primary consumers that produce hydrolytic enzymes to degrade complex plant polymers (cellulose, hemicellulose, lignin, phospholipids, cutin, suberin, protein and others). Depending on the chemical composition of litter, it may take months to years for these macromolecules to be hydrolyzed into metabolizable substrates, including simple monosaccharides, fatty acids, peptides and ions that are metabolized for the biosynthesis of new microorganisms, plant and animal tissues (Fig. 7).
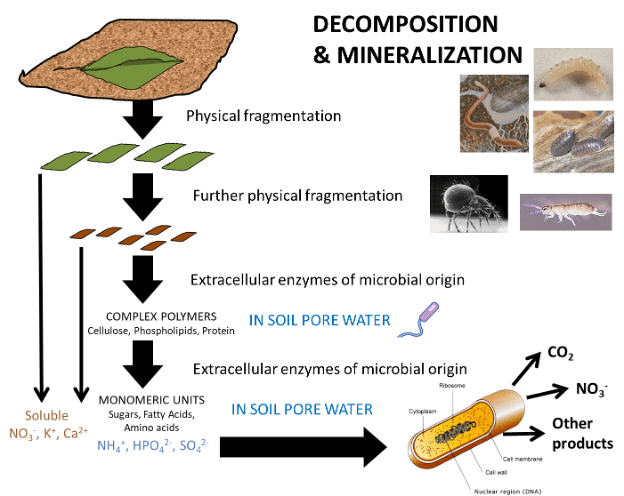
Fig. 7. Decomposition of plant litter in the soil ecosystem. Litter, represented by a senescent leaf, is fragmented physically through the action of soil macro- and mesofauna. Following the rupture of plant cell walls, ions are released from vacuoles and complex organic molecules like cellulose, lignin and proteins enter the soil pore water. Extracellular enzymes produced by soil microorganisms hydrolyse complex compounds into simple monomeric units like sugars, phenols, amino acids and ions. Monomers and ions are then absorbed through the cell membrane of prokaryotes (shown) and eukaryotes (not shown), where they are metabolized through respiration (CO2), to other ionic forms (e.g., NO3- produced by ammonia oxidation and nitrification) and other metabolic byproducts, such as exopolysaccharides for biofilm formation.
Consequently, mass loss during plant litter decomposition is related to its chemistry, where residues with lower lignin content decompose more quickly and decomposition is accelerated by adding nitrogen fertilizer (Fig. 8; Hobbie 2000).
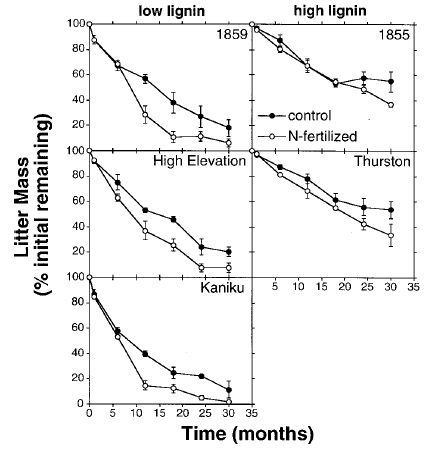
Fig. 8. Decomposition (percent initial mass remaining) of three low-lignin and two high-lignin types of Metrosideros polymorpha litter. Senescent leaf litter was weighed into litterbags (100 cm2 made from 1 mm fiberglass window screen) and placed in control and N-fertilized treatments of a montane wet forest near Thurston Lava Tube, Hawaii Volcanoes National Park, USA. The acetyl bromide lignin content of leaf litter led to their classification as low-lignin: 116 g kg-1 (1859 flow), 131 g kg-1 (High elevation), 105 g kg-1 (Kaniku); or high-lignin: 182 g kg-1 (1855 flow) and 214 g kg-1 (Thurston). From Hobbie S (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494. doi: 10.1007/s100210000042.
Leaf litter in the detritusphere also serves as a model for ecological succession. Fungal decomposer communities show assembly patterns similar to those of plant and animal communities, where different species become dominant at different points in time (Fig. 9). Closely-related fungal species with similar functions are observed to become dominant at the same stage of decomposition, regardless of the habitat. Typically, fungi of the Ascomycota are most abundant during early stages of leaf litter decay and fungi in the Zygomycota become numerically dominant in the late stages of decay (Vivelo and Bhatnagar 2019). Factors affecting fungal succession were decay stage, plant litter type and climate. Fungal preference for growth on decaying leaf litter depends upon their phylum and class, rather than a finer taxonomic classification (e.g., genus), suggesting that fungal succession is related to a generalizable response to resources and climate niches (Vivelo and Bhatnagar 2019). Since fungal succession is one of the most widespread and universal patterns of community assembly and activity in ecosystem ecology, understanding the key taxonomic and biological features of this process provides insight into the energy and carbon fluxes in the detitusphere.
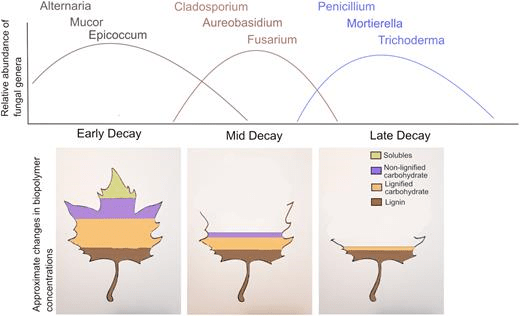
Fig. 9. Hypothesized changes in fungal decomposer community composition during litter decomposition. The concentration of plant biopolymers changes as labile litter chemicals (i.e. soluble carbohydrates) degrade faster than recalcitrant material (i.e. lignin, condensed tannins, waxes and phenolics). Lines on the relative abundance graph represent the change in relative abundance of the corresponding color-coded genera. From Vivelo S, Bhatnagar JM (2019) An evolutionary signal to fungal succession during plant litter decay. FEMS Microbiol Ecol 95(10):fiz145. doi: 10.1093/femsec/fiz145
Porosphere and its ecological significance
Soil is a dynamic porous media, meaning that its physical structure, the chemical reactions and biological activity depend upon the arrangement of the solids and voids in the matrix. Soil voids are often referred to as the porosphere, meaning the pores and their connectivity in a three-dimensional soil volume. The smallest soil pores are nanopores (up to 100 nm dia.) and micropores (<10 μm dia.), whereas cavities larger than 75 μm dia. are designated as macropores. Pores exist within agglomerates, such as the pore space within microaggregates (organo-minerals <250 μm dia.), which is referred to as the aggregatosphere. We chose to define all pores as part of the porosphere (Fig. 10) because pores have general characteristics and functions according to their size. For example, macropores are responsible for preferential soil solution flow and rapid transport of solutes and colloids. Water infiltration and drainage occur more quickly through macropores than other pore types, and lateral water flow in mineral soil horizons depends on macropore connectivity.
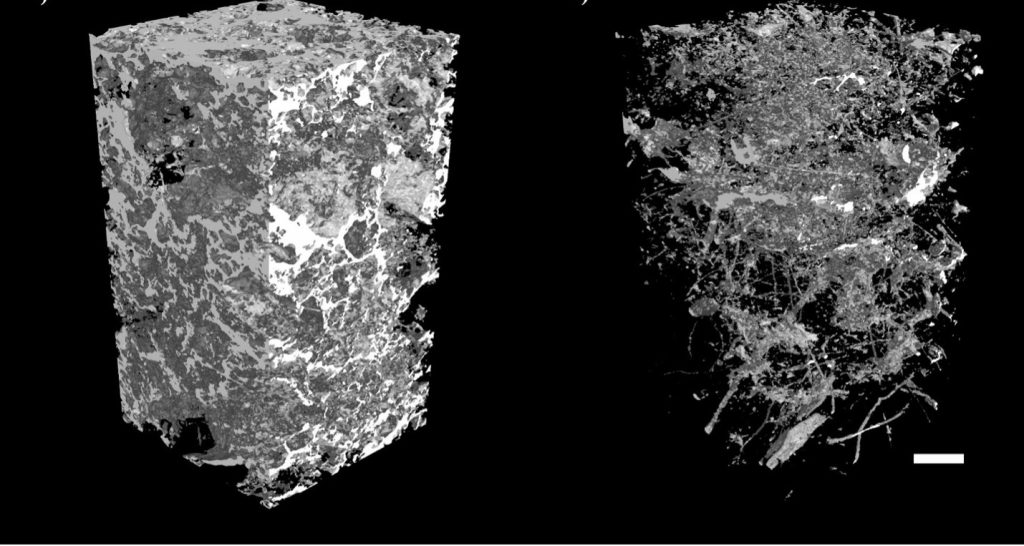
Fig. 10. The porosphere of soil profiles (0–20 cm) from adjacent fields under wheat (Triticum aestivum L.) production in the East Midlands, UK. On the left, tilled soil that was annually ploughed to a depth of 20–25 cm. On the right, zero tilled soil was planted with a seed drill and no cultivation for at least 5 yr. Visualisation of pore space highlights the high connectivity and smaller pores in the tilled soil, versus the presence of numerous biopores in the zero tilled soil. Scale bar = 10 mm. From: Mangalassery S, Sjögersten S, Sparkes D, Sturrock CJ, Craigon J, Mooney SJ (2014) To what extent can zero tillage lead to a reduction in greenhouse gas emissions from temperate soils?. Sci Rep 4:4586. doi: 10.1038/srep04586
Biological life in the soil exists in pores. Plant roots extend into mesopores (from 10-75 μm dia.) and exert pressure that widens these into macropores as roots grow axially and radially in the soil profile. Due to their small size, soil microorganisms live in the micropores (<10 μm dia.), some of which are also inhabited by the tertiary consumers (soil microfauna: protists, nematodes) depending on their body size. Micropores contain more of the soil water, which is favorable to microorganisms and microfauna that live in water films coating soil solids. Micropores connected to larger soil pores permits the dispersal of the soil microorganisms and microfauna into larger pores. From there, microorganisms and microfauna are transported by gravitational forces, water streaming across surfaces through cohesion, and tension-evapotranspiration forces into other compartments, including the rhizosphere, detritusphere and deeper soil layers. Soil microfauna and macrofauna preferentially inhabit the moist, air-filled pores, and create new ones through their digging and burrowing activities. Pores created by earthworms are referred to as the drilosphere (Beare et al 1995), but we consider these macropores to have similar functions as other crevices in the porosphere.
Pore dynamics are related, in part, to the amount of water in the soil matrix. For instance, flooding can cause some soils to swell and occupy more physical space, due to the expansion of clay minerals. As water evaporates, the soil subsides into a smaller volume. Pore connectivity is another dynamic soil property that directs how much water flows vertically and laterally through the soil matrix. Large pores that direct water vertically contribute to the preferential water flow and subsurface drainage. Large and small pores conduct water laterally in the soil profile, which replenishes water in the soil matrix. Besides water, pores also contain air that exchanges between soil and the atmosphere. The chemical composition of soil air depends upon the gas exchange rate plus biological consumption and production of trace gases. Plant roots and many soil fauna are facultative aerobes that need ample oxygen (O2) for their cellular processes and respire carbon dioxide (CO2). For example, root respiration was nearly 100 times greater in a well-aerated moist soil (matric potential < –200 hPa) than an anoxic flooded soil (–10 to–30hPa; Uteau et al., 2015).
The proportion of air and water in soil pores is a key determinant of biological activity, and is affected by soil management. Periodic disturbance of agricultural soil through ploughing alters the pore structure and biological activity. This is because mechanical mixing of the soil structure creates a well-connected network of small-sized pores (Fig. 10). Pore connectivity in this tilled soil increased air exchange while also physically fragmenting and burying plant litter and other detritus, making the soil slightly warmer and drier. Consequently, the tilled soil had 26–31% higher net global warming potential than an adjacent zero tillage systems (Mangalassery et al 2014). This was due primarily to the greater flux of CO2 and methane (CH4) from the tilled soil than the zero tillage system. However, soil water content and microbial biomass were the key determinants of nitrous oxide (N2O) flux because N2O production is related more to soil redox potential than external disturbances. Water-filled pores have greater reducing potential than air-filled pores. Practically, this leads facultative and obligate anaerobic bacteria to use NO3– as an electron acceptor, producing the gases nitric oxide (NO), nitrous oxide (N2O) and dinitrogen (N2) as reaction products. Nitrogen gases that diffuse from the soil ecosystem represent a net loss in the soil nitrogen supply to plants. Furthermore, N2O is a greenhouse gas with an elevated climate forcing effect, relative to CO2. Thus, soil disturbance affecting the porosphere can have far-reaching consequences on trace gas exchanges and plant-available nitrogen levels in the plant-soil system.
Conclusions
The soil ecosystem is a structured environment with interacting abiotic (organic matter, minerals) and biotic components. The biotic activity of soil depends upon both autotrophs, primarily plants, and heterotrophs in the soil foodweb. Primary consumers in the soil foodweb metabolize organic substrates that are released into the rhizosphere or released during the decomposition of detritus. The growth and activity of primary consumers is related by secondary and tertiary consumers (predators), as well as the invertebrate engineers that are responsible for physically fragmenting the coarse litter into fragments that are hydrolyzed to release simple, metabolizable molecules for soil biogeochemical cycling. These processes are responsible for transferring energy, nutrients, trace elements, water and air to other ecosystems on Earth. Interactions are to be expected between biotic components, and these are responsible for self-regulation of the soil ecosystem via feedbacks and homeostatic mechanisms. The ecological diversity and complexity in the soil ecosystem confers stability, which may be manifested in the ability to resist disturbance but allows for regeneration of soil functions following disturbance, due to inherent resilience of the soil ecosystem. Since soil is part of ecology, it is appropriate to study soil pedology and other branches of soil science from an ecological perspective.
Références
Beare MH, Coleman DC, Crossley DA, Hendrix PF, Odum EP (1995). A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. In: Collins HP, Robertson GP, Klug MJ (eds) The Significance and Regulation of Soil Biodiversity. Developments in Plant and Soil Sciences. 63:5–22. Springer, Dordrecht. doi: 10.1007/978-94-011-0479-1_1
Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25(8):468–478. doi: 10.1016/j.tree.2010.05.004
Blagodatskaya E, Kuzyakov Y (2013) Active microorganisms in soil: critical review of estimation criteria and approaches. Soil Biol Biochem 67:192–211. doi: 10.1016/j.soilbio.2013.08.024
Chapin FS III, Eviner VT (2014) Biogeochemical interactions governing terrestrial net primary production. In: Holland HD, Turekian KK (eds) Treatise on Geochemistry, 2nd edn. 10:189–216. Elsevier, Oxford. doi: 10.1016/B978-0-08-095975-7.00806-8
de la Fuente Cantó C, Simonin M, King E, Moulin L, Bennett MJ, Castrillo G, Laplaze L (2020) An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant J 103(3):951–964. doi: 10.1111/tpj.14781.
Fan Y, Miguez-Macho G, Jobbágy EG, Jackson RB, Otero-Casal C (2017) Hydrologic regulation of plant rooting depth. PNAS USA 114(40):10572–10577. doi: 10.1073/pnas.1712381114
Gautrat P, Laffont C, Frugier F, Ruffel S (2021) Nitrogen systemic signaling: From symbiotic nodulation to root acquisition. Trends Plant Sci 26(4):392–406. doi: 10.1016/j.tplants.2020.11.009
Hobbie S (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494. doi: 10.1007/s100210000042
Lajtha K, Bowden RD, Crow S, Fekete I, Kotroczó I, Plante A, Simpson MJ, Nadelhoffer KJ (2018) The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci Total Environ 640–641:1112–1120. doi: 10.1016/j.scitotenv.2018.05.388.
Mangalassery S, Sjögersten S, Sparkes D, Sturrock CJ, Craigon J, Mooney SJ (2014) To what extent can zero tillage lead to a reduction in greenhouse gas emissions from temperate soils?. Sci Rep 4:4586. doi: 10.1038/srep04586
Palmero F, Fernandez JA, Garcia FO, Haro RJ, Prasad PVV, Salvagiotti F, Ciampitti IA (2022) A quantitative review into the contributions of biological nitrogen fixation to agricultural systems by grain legumes. Eur J Agron 136:126514. doi: 10.1016/j.eja.2022.126514.
Rosenblueth M, Ormeño-Orrillo E, López-López A, Rogel MA, Reyes-Hernández BJ, Martínez-Romero JC, Reddy PM, and Martínez-Romero E (2018) Nitrogen fixation in cereals. Front Microbiol 9:1794. doi: 10.3389/fmicb.2018.01794
Shane MW, Lambers H (2005) Cluster roots: A curiosity in context. Plant Soil 274:101–125. doi: 10.1007/s11104-004-2725-7
Sønderholm F, Bjerrum CJ (2021) Minimum levels of atmospheric oxygen from fossil tree roots imply new plant−oxygen feedback. Geobiol 19:250–261. doi: 10.1111/gbi.12435
Sorensen JW, Shade A (2020) Dormancy dynamics and dispersal contribute to soil microbiome resilience. Phil Trans R Soc B 375: 20190255. doi: 10.1098/rstb.2019.0255
Taktek S, St-Arnaud M, Piché Y, Fortin A, Antoun H (2017) Igneous phosphate rock solubilization by biofilm-forming mycorrhizobacteria and hyphobacteria associated with Rhizoglomus irregulare DAOM 197198. Mycorrhiza 27:13–22. doi: 10.1007/s00572-016-0726-z
Uteau D, Hafner S, Pagenkemper SK, Peth S, Wiesenberg GLB, Kuzyakov Y, Horn R (2015) Oxygen and redox potential gradients in the rhizosphere of alfalfa grown on a loamy soil. J Plant Nutr Soil Sci 178:278–287. doi: 10.1002/jpln.201300624
Vivelo S, Bhatnagar JM (2019) An evolutionary signal to fungal succession during plant litter decay. FEMS Microbiol Ecol 95(10):fiz145. doi: 10.1093/femsec/fiz145
Wallace A, Romney EM, Cha JW (1980) Depth distribution of roots of some perennial plants in the Nevada test site area of the northern Mojave Desert. Great Basin Nat Memoir 4:201–207. http://www.jstor.org/stable/23376679
Yazdanbakhsh N, Fisahn J (2010) Analysis of Arabidopsis thaliana root growth kinetics with high temporal and spatial resolution. Ann Bot 105(5):783–791. doi: 10.1093/aob/mcq048




